Answer: the final volume of the hot air balloon is 11341 L
Step-by-step explanation:
The question requires us to calculate the new volume of a hot air balloon, given that the initial volume and temperature were 10000 L and 25°C, and the final temperature is 65°C.
To solve this problem, we can use the following equation, which relates the temperature and volume of a gas under constant pressure:
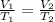
where V1 and T1 are the volume and temperature at the initial state of the system, while V2 and T2 are the final volume and temperature.
Note that we need to apply the temperature in the absolute scale (Kelvin) to use the equation above. Therefore, we can convert the values of temperature given from °C to K as:
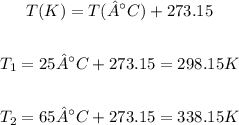
We can calculate the final volume (V2) required using the following rearranged equation:
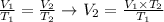
And, applying the values of V1 given by the question and T1 and T2 calculated, we'll have:
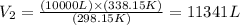
Therefore, the final volume of the hot air balloon is 11341 L.