We have a sum of mixed numbers.
To add them, we can separate the fractional part from the whole part and add the fractional part as normal fractions (looking for a common denominator).
Lastly, we see if we can convert the sum of the pure fractions into a mixed number, if it is greater than 1.
We can do this with this sum as:
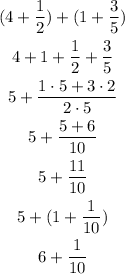
Answer: 6 1/10