Answer:
4 moles of CO₂ and 8 moles of H₂O. Option A is correct
Explanations;
Given the chemical reaction shown below;
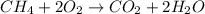
Since 4.0 moles of methane (CH₄) and 8.0 moles of oxygen (O₂) enter this reaction, methane will act as the limiting reactant.
According to stochiometry, 1 mole of methane produces 1 mole of CO₂, hence the moles of CO₂ created at the end of the reaction will also be 4 moles
Also, since 1 mole of methane produces 2 moles of water, the moles of H₂O created at the end of the reaction will be 2(4) = 8 moles.