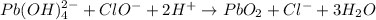Answer:
Explanations:
Given the unbalanced chemical reaction as shown below:
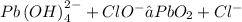
Separate into half reactions
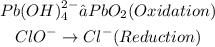
Balance the atoms in each half reactions except hydrogen and oxygen
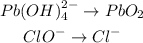
Balance the oxygen atoms by adding water (H2O)
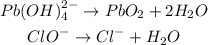
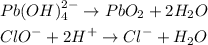
The hydrogen atoms has been balanced in the last equation
Balance the charges
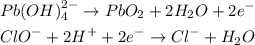
Cancel the charge and add the half reactions to have: