Answer
The concentration in parts per million (ppm) = 332,000 ppm.
Step-by-step explanation
Given:
Mass of salt solute = 332 grams
Mass of water solvent = 1000 grams
What to find:
The concentration in parts per million (ppm).
Solution:
The concentration in parts per million (ppm) formula is:
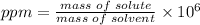
Plugging the values of the given parameters into the formula:
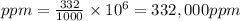
The concentration in parts per million (ppm) = 332,000 ppm.