Answer: there are 1.0 x 10^4 moles of (NH4)2Cr2O7 in 0.025g of this compound (letter B).
Step-by-step explanation:
The question requires us to determine the number of moles in 0.025 g (NH4)2Cr2O7.
To solve this problem, we need to determine the molar mass of (NH4)2Cr2O7 and then use this value to calculate the amount of moles required.
The atomic masses of nitrogen, hydrogen, chromium and oxygen are:
- N = 14.01 amu
- H = 1.008 amu
- Cr = 51.99 amu
- O = 15.99 amu
To calculate the molar mass, we need to consider the number of atoms of each element and their respective atomic mass. We can calculate the molar mass of (NH4)2Cr2O7 as it follows:
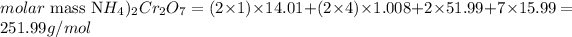
From the molar mass calculated (251.99 g/mol), we know that there are 251.99g of (NH4)2Cr2O7 in 1 mol of it. Thus, we can calculate the amount of moles in 0.025g of the compound as:
251.99 g ------------------------- 1 mol
0.025 g -------------------------- x
Solving for x, we'll have:
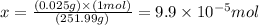
Therefore, there are 9.9 x 10^-5 moles, which is approximately 1.0 x 10^-4 moles of (NH4)2Cr2O7 in 0.025g of this compound.