Answer:
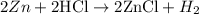
Explanations:
According to the question, we are balancing the equation between the zinc element and hydrochloric acid as shown:
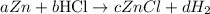
Next is to balance the chemical reaction by determining the coefficient
For the zinc element:
a = c
For the hydrogen element:
b = 2d
For the Chlorine element
b = c
Recall that d = b/2 = c/2
Substitute the coefficient into the reaction in terms of "c"
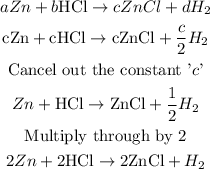
This gives a balanced chemical reaction for the reaction