Applying the Pythagorean theorem, with a = 6 and b = 7 (the legs of the right triangle formed), the length of the hypotenuse c is:
![\begin{gathered} c^2=a^2+b^2 \\ c^2=6^2+7^2 \\ c^2=36+49 \\ c^2=85 \\ c=\sqrt[]{85} \end{gathered}](https://img.qammunity.org/2023/formulas/mathematics/college/vu6ysxjmur6nvtgmscdgo83w1o1x9s0wtx.png)
By definition:
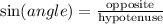
In this case, the angle is θ, the hypotenuse is c and the opposite side is 7 units long. Substituting this information into the equation, we get:
![\sin \theta=\frac{7}{\sqrt[]{85}}](https://img.qammunity.org/2023/formulas/mathematics/college/t1jlinlbw637xoq11zl5db9qhkbtfgf5c7.png)