INFORMATION:
We know that:
- pure water boils at 99.8 degrees celcius
And we must calculate the expected elevated boiling point of a solution of 2.50g of CaCl2, in 50.0mL (i.e., 50.0g) of H2O
STEP BY STEP EXPLANATION:
To calculate it, we need to use that:
Boiling point of solution = boiling point of pure solvent + boiling point elevation (ΔTb)
The elevation in boiling point (ΔTb) is proportional to the concentration of the solute in the solution. It can be calculated via the following equation.
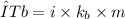
Where,
- i is the Van’t Hoff factor
- Kb is the ebullioscopic constant
- m is the molality of the solute
From given information, we know that:
- i = 3
Now, the ebullioscopic constant (Kb) is often expressed in terms of °C * kg * mol^-1. The value of Kb for water is 0.512.
So, kb = 0.512 °C * kg * mol^-1
Then, we must calculate the molality
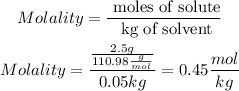
So, m = 0.45 mol/kg
Replacing the values in the formula for ΔTb
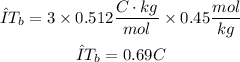
Finally, the expected elevated boiling point of the solution would be
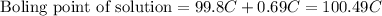
ANSWER:
The expected elevated boiling point of the solution is 100.49 °C