Answer:
Explanations:
An acid is a substance that gives up hydrogen ions in solution or dissociates in solution.
A base is a substance that releases hydroxide ion in aqueous solution while an amphoteric substance is a substance that act as an acid in presence of a base and act as a base in presence of an acid.
From the given table, the following compounds are acids.
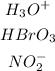
The bases are as shown;
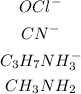
The amphoteric compounds are;
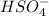
For the conjugates
The conjugate acid of CGH3NH2 is CH3NH3+. The compound has no conjugate base.
The conjugate base of HSO4- is SO4^2- and H2SO4 is its conjugate acid.
The conjugate acid of CN- is HCN. The cyanide ion has no conjugate base.
The conjugate acid of NO2- is HNO2. It has no conjugate base