Answer:
0.181 M
Step-by-step explanation:
Begin by finding the total moles by using the volume and molarity given:
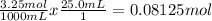
(Remember molarity is mol/L, which is equal to mol/1000 mL.)
Divide the moles by the volume after dilution:
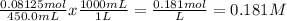
The concentration of the diluted solution is 0.181 M.