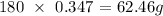Answer:
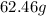
Step-by-step explanation:
Here, we want to get the number of grams of sugar in 0.347 moles of sugar
In 1 mole of sugar,the number of moles present equals the molar mass of sugar
the molar mass of sugar is 180 g/mol
Thus, the number of grams in 0.347 moles will be the product of the molar mass and the number of moles
Mathematically,we have this as: