The difference between a mole of propane and a gram of propane is that A mole of propane has more mass than a gram of propane. The correct answer is option C.
A mole is a unit used in chemistry to express the amount of a substance, and it contains Avogadro's number of entities, which is approximately 6.02 x
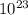 . The molar mass of propane (
. The molar mass of propane (
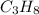 ) is the sum of the molar masses of its constituent atoms. In this case, carbon has a molar mass of 12 grams per mole, and hydrogen has a molar mass of approximately 1 gram per mole. Therefore, the molar mass of propane is (3 × 12) + (8 × 1) = 36 + 8 = 44 grams per mole.
) is the sum of the molar masses of its constituent atoms. In this case, carbon has a molar mass of 12 grams per mole, and hydrogen has a molar mass of approximately 1 gram per mole. Therefore, the molar mass of propane is (3 × 12) + (8 × 1) = 36 + 8 = 44 grams per mole.
Comparatively, a gram of propane refers to a mass of 1 gram of the substance. The difference lies in the quantity being measured. While a mole of propane contains Avogadro's number of molecules (6.02 x
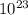 ), a gram of propane represents a fixed mass of 1 gram. Therefore, a mole of propane has more mass than a gram of propane.
), a gram of propane represents a fixed mass of 1 gram. Therefore, a mole of propane has more mass than a gram of propane.