ANSWER
The final pressure of the gas is 545.03 torr
Step-by-step explanation
Given that;
The initial volume of the gas is 856 cm^3
The initial pressure of the gas is 661 torr
The initial temperature of the gas is 25 degrees Celcius
The final temperature of the gas is 60 degrees Celcius
The final volume of the gas is 1160 cm^3
To find the final pressure of the gas, follow the steps below
Step 1; Write the general gas law
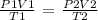
Step 2; Convert the temperatures to degrees kelvin
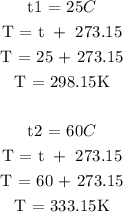
Step 3; substitute the given data into the formula in step 1

Therefore, the final pressure of the gas is 545.03 torr