First, we have to write the equation:
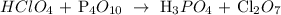
Then, we have to balance it:
![12HClO_4+\text{ P}_4O_(10)\operatorname{\rightarrow}\text{ 4H}_3PO_4\text{ + 6Cl}_2O_7]()
After this, we have to make the respective calculations.
a. The first one is the theoretical yield. We have to calculate the molecular weight of each substance involved in the exercise:
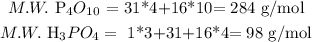
Then, we calculate the produced quantity of phosphoric acid, in theory:

Then, the answer is that the theoretical amount produced of phosphoric acid is 9.524 g.
b. Now, we have to apply the following formula:
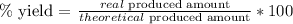
As we have those values, we replace them:
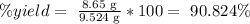
The answer is that the yield equals 90.824%