1) List the known quantities
Initial conditions
Concentration 1: 1.6 M
Volume 1: 175 mL
Final conditions
Volume 2: 1 L
Concentration 2: unknown
2) Set the equation
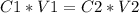
3) Convert units
Volume
1 L = 1000 mL
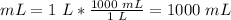
4) Plug in the known quantities and solve for C2
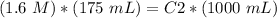
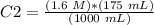
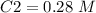
The new concentration is 0.28 M.
.