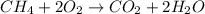We have the next skeletal equation
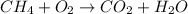
And we must balance it.
To balance it we need to know that
By balancing chemical reactions we seek to comply with the Law of Conservation of Matter. According to this law, during any physical or chemical change, the total mass of the products remains equal to the total mass of the reactants.
Then, knowing that the balanced equation would be
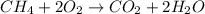
ANSWER: