They ask us to make a solution starting from a solution with a higher concentration, to find the final volume we will apply the following relationship of concentration and volume:
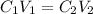
Where,
C1 is the initial concetration, 6.00M
V1 is the initial volume, 24mL
C2 is the final concentration, 1.50M
V2 is the final volumen, unknown
We clear V2 and replace the known data:
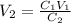
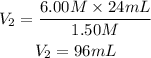
The solution has to be diluted until a volume equal to 96mL
Answer: 96mL