First, we have to remember the equation to calculate the heat of evaporation:
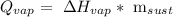
Q is the heat, ΔH is the vaporization heat of the substance, and m is the mass.
If we have the vaporation heat in terms of moles (as in this case), we have to multiply it by the number of moles instead of the mass. For that purpose, we have to calculate the molecular weight of the water:
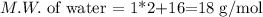
Then, we can pass the grams to moles:
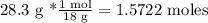
And we can finally calculate the heat:
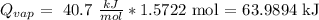
The answer is that the necessary heat to evaporate the water is 63.9894 kJ approx.