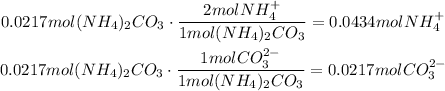The first step is to determine how many moles of each ion are there in one mole of (NH4)2CO3.
In one mole of (NH4)2CO3, there are 2 moles of NH4 + and 1 mole of CO3 2-.
Find the number of moles that are present in 36.5mL of solution by multiplying this volume times the concentration of the solution. First convert the mililiters to liters (1L=1000mL):
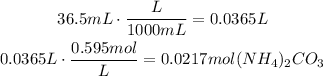
Finally, use the amounts of moles of each ion per mole of substance (determined at the beginning) to find the amount of moles of each ion present in the given volume: