ANSWER
The number of moles of aluminum chloride is 1.533 moles
Explanation:
Given information
Number of moles of Magnesium chloride = 2.3 moles
The next thing is to write the balanced equation of the reaction
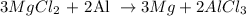
From the balanced chemical reaction equation, you will see that 3 moles of Magnesium chloride react with 2 moles of aluminum to produce 3 moles of magnesium and 2 moles of aluminum chloride.
The next step is to find the number of moles of aluminum chloride in the reaction using a stoichiometry ratio.
Let x represents the number of moles of aluminum chloride
From the chemical reaction equation,
3 moles of magnesium chloride give 2 moles of aluminum chloride
2. 3 moles of magnesium will give x moles of aluminum chloride
The above statement can be translated below as
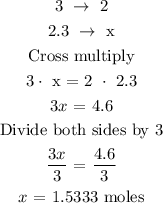
Since x represents the number of moles of aluminum chloride, therefore, the number of moles of aluminum chloride is 1.533 moles