Given:
The volume of alcohol, V_a=55 mL
The density of the alcohol, ρ_a=0.75 g/mL
The mass of the water in the mixture, M_w=25 g
The density of water, ρ_w=1.00 g/mL
To find:
The density of the mixture.
Step-by-step explanation:
The mass of the alcohol in the mixture is given by,
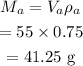
The volume of the water in the mixture,
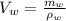
On substituting the known values,
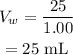
Thus the density of the mixture is given by,
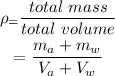
On substituting the known values,
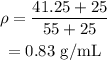
Final answer:
Thus the density of the mixture is 0.83 g/mL