The question provides a table with the atomic mass of a few elements (beryllium, copper, cadmium, calcium and iodine) and requires us to calculate the molar mass of the salts BeI2, CuI2, CdI2 and CaI2.
The molar mass of a compound is calculated considering the atomic mass of the elements that compose the molecule and the amount of atoms of each of these elements. Considering the hypothetical compound AX2, its molar mass would be calculated as:
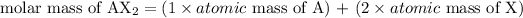
Since the question provided the atomic mass of the elements, we can calculate the molar mass of the salts given in a similar way as the equation above for AX2.
- For BeI2:
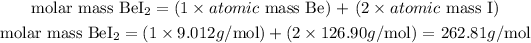
The molar mass of BeI2 is 262.81 g/mol.
- For CuI2:
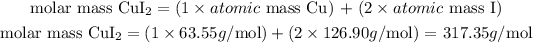
The molar mass of CuI2 is 317.35 g/mol.
- For CdI2:
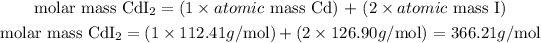
The molar mass of CdI2 is 366.21 g/mol.
- For CaI2:
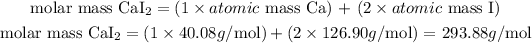
The molar mass o CaI2 is 293.88 g/mol.