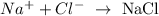Explanation
Sodium chloride (NaCl) exists in a solid state cause it possesses a high melting point. NaCl has a strong force attraction between sodium ion and chloride ion and this invole the transferring of electrons from sodium to chloride. Therefore, sodium chloride exists in the solid state.