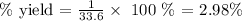Answer:
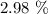
Step-by-step explanation:
Here, we want to get the percentage yield
Mathematically, we have that as:
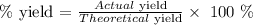
We have the actual yield, now let us calculate the theoretical yield
We start by writing the complete equation of the reaction
We have that as:
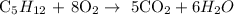
In a combustion reaction, the hydrocarbon which in this case methane is usually the limiting reactant
Thus, we can calculate the mass of water produced by using the mass of Pentane given
Firstly, we need to get the number of moles of pentane that reacted
To get that, we have to divide the mass of pentane given by the molar mass of pentane
The molar mass of pentane is 72 g/mol
Thus, we have the number of moles as:
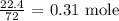
From the equation of reaction:
1 mole of pentane produced 6 moles of water
0.311 mole of pentane will produce:
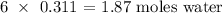
Now, to get the mass of water produced
We multiply this number of moles by the molar mass of water
The molar mass of water is 18 g/mol
That means the mass theoretically produced will be:
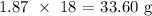
From here, we have the percentage yield as: