ANSWER
The final pressure of the gas is 626 mmHg
Step-by-step explanation
Given that;
The initial temperature of the gas is 323K
The final temperature of the gas is 273.15K
The original pressure of the container is 740.0mmHg
Follow the process below to find the final pressure of the gas
The provided that shows that the volume remains constant, and this process is called Isochoric.
Step 1: Write the gas law equation at constant volume
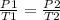
Step 2: Substitute the given data into the formula in step 1 to find P2
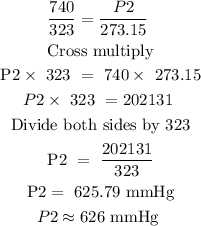
Hence, the final pressure of the gas is 626 mmHg