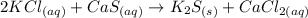Answer
Part A:
Given equation:
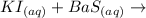
The above equation is an example of a double replacement reaction. A double replacement (or double displacement) reaction is a reaction in which the positive and negative ions of two ionic compounds exchange.
Note: The oxidation number of:
K = +1
I = -1
Ba = +2
S = -2
So, the complete chemical equation including phases of the given reaction is:
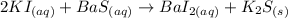
Part B:
Given equation:
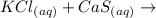
Also, the equation is an example of a double replacement reaction.
Note: The oxidation number of:
K = +1
Cl = -1
Ca = +2
S = -2
The complete chemical equation including phases of the given reaction is: