To answer this question we have to use Avogadro's number, that is the number of molecules present in one mole of any substance.
According to it, there are 6.022x10^23 molecules of copper in one mole of it:
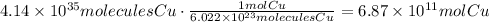
It means that there are 6.87x10^11 moles of copper.