Answer:
24.9 grams of potassium iodide.
Step-by-step explanation:
We have the molarity of KI solution which is 0.075 M and the volume which is 2 liters (L). Based on these data, we can find the number of moles of KI, by using the molarity formula:
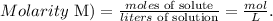
We can solve for moles of solute, like this:
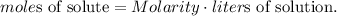
And then, we replace the values that we have in the new formula:
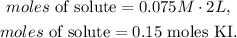
Finally, we have to do the conversion from 0.15 moles of KI to grams using its molecular weight which you can see in the statement of the question (166 g/mol). The conversion will be:
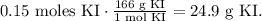
We need 24.9 grams of potassium iodide to make 2 liters of a 0.075 M KI solution.