Answer:
3.8grams. Option D is correct
Explanations:
Given the chemical reaction below as shown;
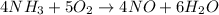
Given the following parameter
Mass of NH3 = 2.4grams
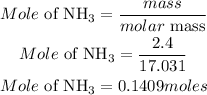
Accoridng to stoichiometry, 4 moles of NH3 produces 6 moles of H2O, therefore the moles of water required will be expressed as:
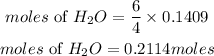
Calculate the mass of water (H2O)
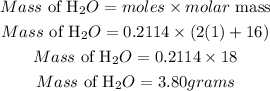
Therefore the mass in grams of H2O that will be produced if you react 2.4 grams of NH3 is 3.8grams