Let's see that 3 moles of magnesium chloride (MgCl2) produce 2 moles of aluminum chloride (AlCl3), we can do a rule of three to find the number of moles required of AlCl3 by 2.1 moles of MgCl2, like this:
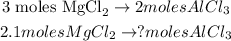
The calculation would be:
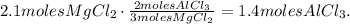
So, the answer is that 1.4 moles of aluminum chloride are produced by 2.1 moles of magnesium chloride.