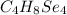Answer:
Step-by-step explanation:
Here, we want to get the molecular formula of the substance
We start by dividing the percentages by the atomic masses of the individual elements
The atomic mass of Selenium is 79 amu
The atomic mass of Carbon is 12 amu
The atomic mass of hydrogen is 1 amu
From the given percentages, to get the percentage for hydrogen, we subtract the percentages of carbon and selenium
We have that as 100- (12.9 + 84.9) = 2.2%
We have the calculation as follows:
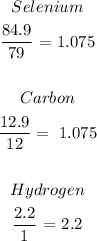
From the above, we can see that the minimum value is 1.075
Now, we divide the values by the smallest
We have that as:
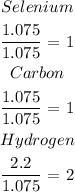
We can see that the empirical formula is thus:
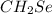
Now, let us get the molecular formula
We have to find the mass of the empirical formula, then divide the molar mass by it
This will give us the required multiple needed to get the molecular formula from the empirical formula
Mathematically, we have that as:
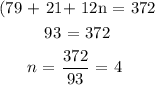
This means that the molecular formula is: