Answer:
9moles of N2
Explanations:
Given the reaction represented by the equation:
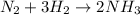
Given the following parameters
• Moles of ammonia = 18 moles
According to stoichiometry, 1 mole of nitrogen produces 2 moles of ammonia. Therefore the moles of nitrogen required is given as:
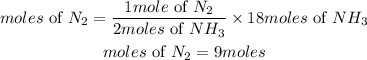
Hence the moles of nitrogen required to produce 18 mol of ammonia is 9moles