Answer: 291.3 g of CaCl2 would be necessary to prepare the solution given. The best option to answer the question is the last one (letter E, 291 g)
Step-by-step explanation:
The question reuquires us to determine the mass of CaCl2 necessary to prepare 0.00175× 10^6 mL of a 1.50 M solution.
We can use the following equation, which corresponds to the definition of molarity, to calculate the number of moles of CaCl2 necessary to prepare the solution:
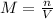
Where M is the molarity of the solution (given in mol/L or M), n is the number of moles of solute (in mol) and V is the volume of solution (in L).
Note that we'll need to convert the volume given (0.00175× 10^6 mL) from mL to L:
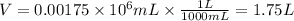
Next, we can rearrange the equation for molarity in order to calculate the number of moles of CaCl2:
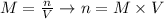
And, applying the values given by the question:
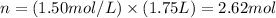
Therefore, 2.62 moles of CaCl2 would be necessary to prepare the solution.
We can use the following equation to convert the number of moles calculated (2.62 mol) to the mass required, considering the molar mass of CaCl2 given (MM = 110.98 g/mol):
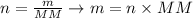
Where n corresponds to the number of moles (in mol), m is the mass of the sample (in grams) and MM corresponds to the molar mass of the compound (in g/mol).
Applying the molarity given by the question and the number of moles calculated, we'll have:
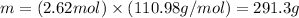
Therefore, 291.3 g of CaCl2 would be necessary to prepare the solution given. The best option to answer the question is the last one (letter E, 291 g).