Answer:
-239.13kJ/mol
Explanations:
The complete chemical reaction between the sodium metal and hydrochloric acid is expressed as shown:
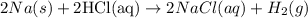
The enthalpy of the reaction is expressed using the formula;
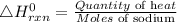
Given that quantity of energy released is -2090J (Since energy is released by the system)
Get the moles of sodium

Next is to get the required enthalpy of the reaction in kJ/mol
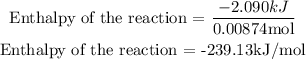
Hence the enthalpy of the reaction is -239.13kJ/mol