Given:
The final amount is given as A = ₱5,000.
The number of years is T = 4.
The number of times compounded is each month, n = 12 per year.
The rate of interest is r = 12% = 0.12.
The objective is to find the amount to be deposited.
Step-by-step explanation:
The general formula to calculate the principal amount is,
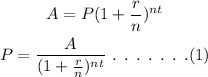
On plugging the given values in equation (1),
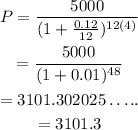
Hence, the amount to be deposited is ₱3101.3