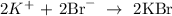Answer:
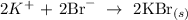
Step-by-step explanation:
Here, we want to write the net ionic equation for the given equation;
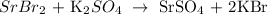
Now, what is left is to write the participating ions:
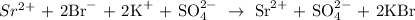
We must identify that the Potassium Bromide is the precipitate and thus would not be dissociated into ions
Thus, we have it that:
The Strontium ion cancels out, including the sulphate
We have left: