Answer
61 g/L
Step-by-step explanation
The first step is to calculate the mass of NaCl in 4.0 g/L solution.
As 1000 ml NaCl solution contains 4 grams of NaCl
So, 200 ml NaCl solution contains:
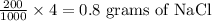
Also, we have to calculate the mass of another NaClin 8% NaCl solution (m/v).
As 100 ml NaCl solution contains 8 grams of NaCl
So, 600 ml NaCl solution contains:
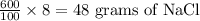
Hence, the total mass of NaCl in the final concentration = 0.8 + 48 = 48.8 g
Also, the total volume of NaCl solution = 200 + 600 = 800 mL = 0.8 L
The final step is to calculate the final concentration of salt in the solution using the formula below:
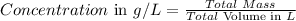
So,
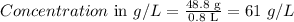
Therefore, the final concentration of salt in the solution is 61 g/L