Answer:
99.93kJ
Explanations:
Given the chemical reaction expressed as:
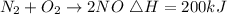
From the reaction, 1 mole of nitrogen absorbs 200kJ of heat.
Determine the mole of nitrogen
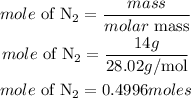
The amount of energy 0.4496moles of N2 will absorb is given as:
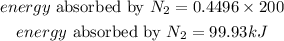
Hence the amount of heat that will be absorbed when 14 grams of Nitrogen reacts with excess O2 is 99.93kJ