ANSWER
Step-by-step explanation
Given that;
The atomic mass of chlorine = 35
The % of the sample in chlorine atom = 75.53%
Relative atomic mass is a physical quantity that define the ratio of the average mass of atoms of a chemical in a given sample to the atomic mass constant.
The next step is to find the relative atomic mass of the sample
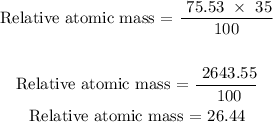
Therefore, the relative atomic mass is 26.44