Answer
Step-by-step explanation
Given that:
Mass of nickel (II) bromide (NiBr₂) that dissolved = 24.9 g
The volume of the aqueous solution of potassium carbonate (K₂CO₃) = 300 mL = 0.300 L
The molarity of K₂CO₃ = 0.60 M
What to find:
The final molarity of nickel(II) cation in the solution.
Step-by-step solution:
The first step is to write the balanced chemical equation for the reaction.
NiBr₂ + K₂CO₃ → NiCO₃ + 2KBr
The next step is to calculate the moles of NiBr₂ using:
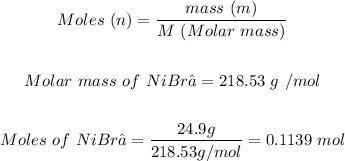
Also, the moles of K₂CO₃ can be calculated using:
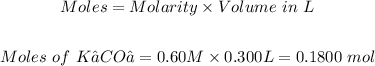
Using the mole ratio of NiBr₂ to K₂CO₃ from the equation above, that is (1:1)
we can say that K₂CO₃ is in excess.
So 0.1139 mol NiBr₂ gets converted into NiCO₃.
Hence, the final molarity of nickel(II) cation in the solution can be calculated using:
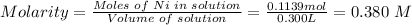
Hence, the of nickel(II) cation in the solution is 0.380