Answer
0.200 M HCl solution
Explanation
Given:
Volume of NaOH, Vb = 28.6 mL
Molarity of NaOH, Cb = 0.175 M
Volume of HCl , Vₐ = 25.0 mL
What to find:
The molarity of the HCl solution, Cₐ.
Step-by-step solution:
Step 1: Write the balance chemical equation for the reaction
2NaOH + 2HCl ------> 2NaCl + H₂O
Step 2: To calculate the molarity of the HCl solution.
Using the formula below:
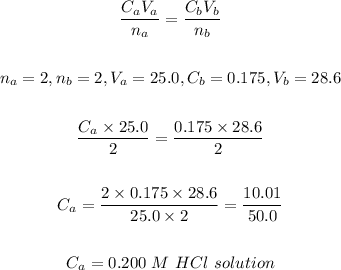
The molarity of the HCl solution = 0.200 M.