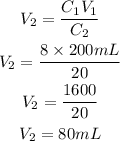Answer:
Explanations:
The formula for calculating the dilution of a solution is given as:
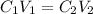
where
C is the concentration of ammonia
V is the volume of solution
Convert the percent by weight of ammonia to concentration to have:
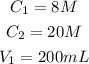
Required
Final volume V2
Substitute the given parameters into the formula to have: