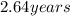Step-by-step explanation:
The dunction is given below as
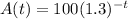
To figure out the half life,
The new mass of the decayed substance will be half that of the initial substance
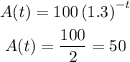
By substituting the values, we will have
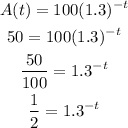
Apply exponent rules
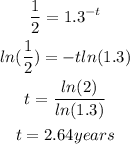
Hence,
The final answer is