In the chemical reaction, you can see that 1 mol of O2 reacts with 2 moles of CO, so the number of moles of CO will be this number multiplied by two. You can see this better, like this:
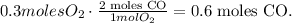
Using this number and the molar mass of CO, which you can calculate using the periodic table ( 16 g/mol + 12 g/mol = 28 g/mol CO), we're going to obtain the mass of CO:
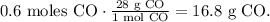
We need 16.8 grams of CO to complete the reaction with 0.3 moles of O2.