The question requires us to identify how many atoms of H are in the notatio 6 Fe(CH3COO)3.
When analyzing molecular formulas, we need to keep the following rules in mind:
- the number right after an element indicates the number of atoms of that element (when there isn't a number, we can consider the number of atoms = 1)
- when there are parenthesis in the formula, the number right after the last parenthesis must multiplicate the numbers inside of it. For example: (OH)3 means that there are 3*1 O atoms and 3*1 H atoms; (CH2)3 means that there are 3*1 C atoms and 2*3 H atoms
With these rules in mind, we can analyze the formula given:
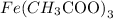
Inside the parenthesis, we have "H3", and outside the parenthesis, there is the number 3 (indicating that the whole structure inside the parenthesis repeats three times in the molecule).
Thus, we can calculate the number of H atoms in one molecule as it follows:
atoms of H = 3 * 3 = 9 atoms in one molecule
Since the notation provided by the question was "6 Fe(CH3COO)3", we can understand that there are six molecules of Fe(CH3COO)3 because of the number 6 before the molecular formula.
Thus, we must multiply the number of H atoms in one molecule by six to obtain the total number of H atoms in the notation:
total atoms of H = 9 * 6 = 54 atoms of H in the notation
Therefore, the notation given by the question indicates that there are 54 atoms of H.