Answer:
-5965.9 kJ.
Step-by-step explanation:
What is given?
Molar enthalpy of combustion of glucose (C6H12O6) = -2538.7 kJ/mol.
Moles of glucose = 2.35 moles.
Steb-by-step solution:
Based on the molar enthalpy and the moles, we can do a dimensional analysis to find the amount of energy. Remember that kJ is the unit for energy, so the dimensional analysis will look like this:
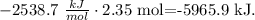
The amount of energy produced by 2.35 mol of glucose is -5965.9 kJ.