Step 1 - Find the molar mass of the substance
To calculate the molar mass of a molecule, we need to multiply the molar mass of each atom by how many times it appears in the molecule. In this case, NH3, N appears only once whereas H appears three times. The molar mass (M) of NH3 will be thus:
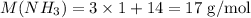
Step 2 - Finding the percent of N in NH3
Since part of the molar mass comes from N (14 g/mol), we can assume that 17 g/mol represent 100%. We can set thus the following proportion:
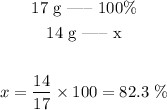
The percentage of N in NH3 corresponds thus to 82.3%