Given:
Diffusion constant = 1.71 x 10⁻⁶ m²/s.
Let's find the average distance a perfume molecule moves in air.
Apply the formula:
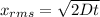
Where:
D is the diffusion constant
t is the time = 1 second.
Thus, we have:
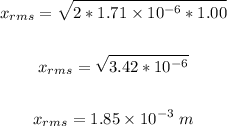
Therefore, the average distance a perfume molecule moves in one second in air is 1.85 x 10⁻³ m
ANSWER:
1.85 x 10⁻³ m