Answer:
a. The limiting reagent is NO2.
b. 126g of nitric acid can be produced.
Step-by-step explanation:
1st) It is necessary to write the balanced equation:
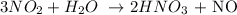
From the balanced equation we know that 3 moles of NO2 (3x46g= 138g) react with 1 mol of water (18g/mol) to produce 2 moles of nitric acid (2x63g= 126g) and 1 mol of NO (30g/mol).
2nd) To calculate the limiting reagent it is necessary to use the given values and the stoichiometry of the balanced equation:
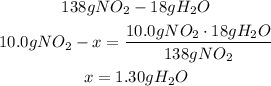
The 10.0g of NO2 will need 1.30g of H2O to react.
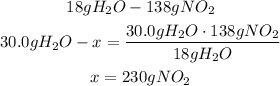
The 30.0g of H2O will need 230g of NO2 to react.
So, as we only have 10.0g of NO2 and 30.0g of H2O, the limiting reagent will be NO2.
3rd) Now, to calculate the theorical yield, we need to use the stoichiometry of the balanced equation using the limiting reagent:
1 mol of H2O produces 2 moles of nitric acid. With the molar mass of nitric acid (63g/mol), we can calculate the grams.
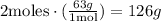
Finally, 126g of nitric acid will be produced if the reaction is 100% efficient (theoretical yield).